Early Recognition of Digoxin Toxicity Is Essential
Digoxin Toxicity May Be Difficult to Recognize1
Digoxin toxicity symptoms are often nonspecific and may be similar to those commonly observed as part of the normal aging process1
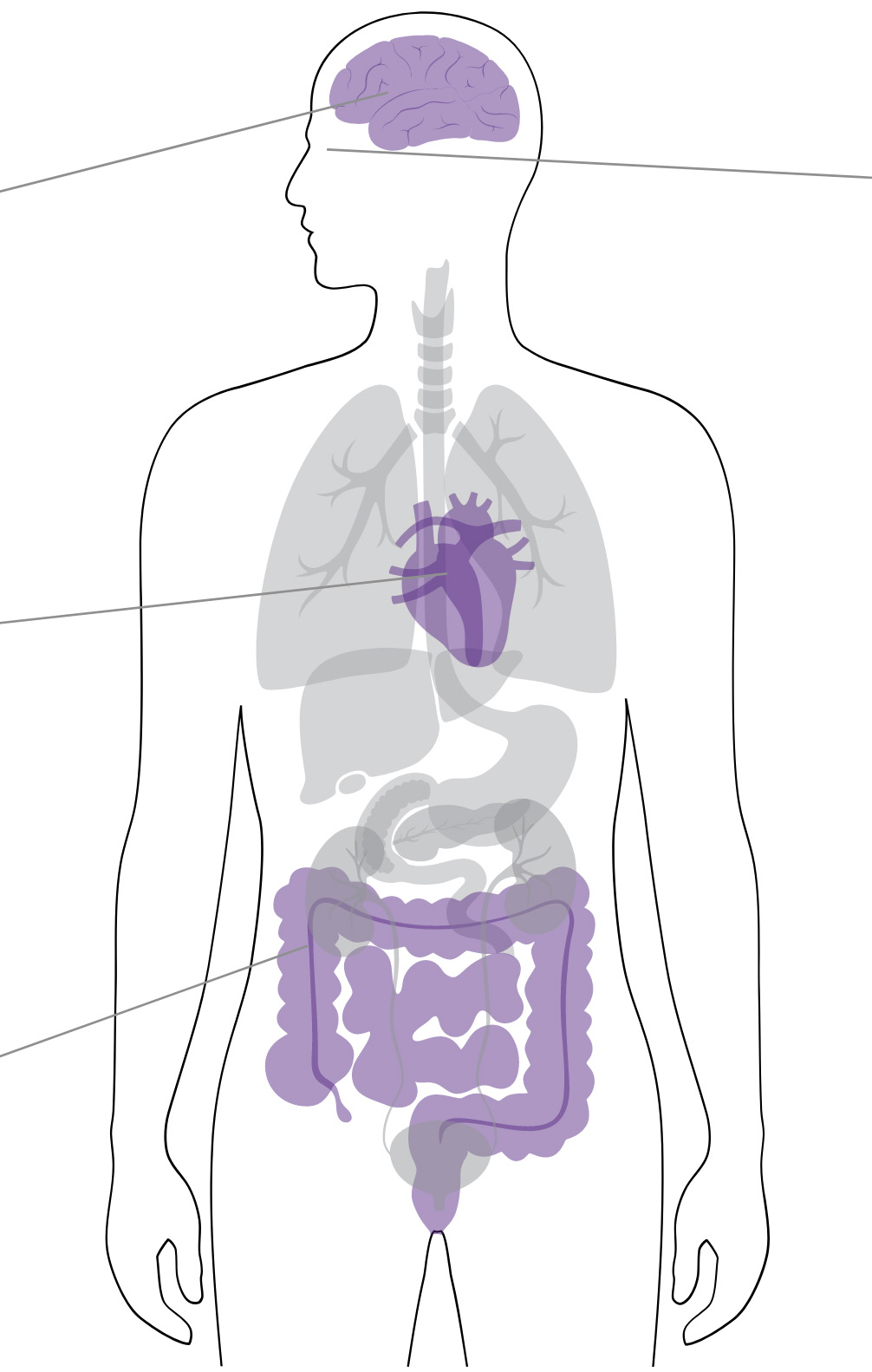
Neurologic symptoms
Headache, confusion, altered mental status, vertigo, trigeminal neuralgia and other neuralgias, convulsions, paresthesia, delirium, psychosis, hallucinations1
Cardiac symptoms
Worsening of heart failure, atrial arrhythmias, AV block, ventricular arrhythmias, AV nodal arrhythmias1
Gastrointestinal symptoms
Anorexia, nausea, vomiting, diarrhea, abdominal pain, constipation, intestinal ischemia1
Visual symptoms
Blurring, color changes: particularly green or yellow hues with possible halos, rarely: scotomas micropsia, macropsia, amblyopias1
General symptoms
Fatigue, malaise, insomnia, loss of interest, depression, anxiety, restlessness, weakness1
Other rare symptoms
Urticaria, eosinophilia, thrombocytopenia, gynecomastia, maculopapular rash, hyperkalemia1
Any Indication of Potentially Life-Threatening Digoxin Toxicity May Necessitate the Need for Immediate Intervention.
Signs of potentially life-threatening digoxin toxicity include:
Cardiac Dysrhythmias3,4
- Severe ventricular dysrhythmias
- Progressive bradydysrhythmias
- Ventricular tachydysrhythmias are more common in chronic or late acute digoxin toxicity5
- Early bradydysrhythmias in acute toxicity are often responsive to atropine5
- Bradydysrhythmias in chronic or late acute digoxin toxicity are often minimally responsive to atropine5
Laboratory Parameters2,3
- Severely elevated serum potassium concentrations with rapidly progressive signs and symptoms
- Severely elevated serum digoxin concentrations with clinically significant signs and symptoms
- Hyperkalemia (e.g., >5.5 mEq/L in adults or >6 mEq/L in children) frequently occurs in acute digoxin toxicity and is an important predictor of mortality3,5
- Severely elevated digoxin levels (e.g., steady-state concentrations >6 ng/mL in adults or >4 ng/mL in children) can lead to potentially life-threatening toxicity3
Evidence of End-Organ Dysfunction2
- Signs and symptoms of end-organ dysfunction from hypoperfusion
- Signs and symptoms include renal failure, altered mental status, abdominal pain2

Any ONE
may indicate the need for
IMMEDIATE INTERVENTION2,3

